A Person Begins To Feel Cooler When A Breeze Moves Over The Skin. Which Mechanism Is Involved?
Temperature and Heat
7 Mechanisms of Oestrus Transfer
Learning Objectives
By the end of this section, yous will be able to:
- Explain some phenomena that involve conductive, convective, and radiative oestrus transfer
- Solve problems on the relationships between heat transfer, time, and rate of heat transfer
- Solve problems using the formulas for conduction and radiation
Just every bit interesting as the effects of heat transfer on a organization are the methods by which it occurs. Whenever there is a temperature difference, rut transfer occurs. It may occur chop-chop, as through a cooking pan, or slowly, every bit through the walls of a picnic water ice breast. So many processes involve estrus transfer that it is hard to imagine a situation where no estrus transfer occurs. Yet every heat transfer takes place past just iii methods:
- Conduction is heat transfer through stationary affair by physical contact. (The matter is stationary on a macroscopic scale—we know that thermal motion of the atoms and molecules occurs at any temperature above accented zippo.) Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction.
- Convection is the heat transfer past the macroscopic movement of a fluid. This type of transfer takes place in a forced-air furnace and in weather systems, for instance.
- Heat transfer by radiation occurs when microwaves, infrared radiation, visible light, or some other class of electromagnetic radiations is emitted or absorbed. An obvious example is the warming of Earth by the Sun. A less obvious example is thermal radiation from the human being body.
In the illustration at the get-go of this chapter, the fire warms the snowshoers' faces largely past radiation. Convection carries some heat to them, but about of the air catamenia from the fire is upward (creating the familiar shape of flames), carrying rut to the food being cooked and into the sky. The snowshoers wear clothes designed with low electrical conductivity to prevent heat flow out of their bodies.
In this department, we examine these methods in some detail. Each method has unique and interesting characteristics, merely all three have two things in common: They transfer heat solely because of a temperature difference, and the greater the temperature divergence, the faster the heat transfer ((Figure)).
In a fireplace, heat transfer occurs past all three methods: conduction, convection, and radiations. Radiations is responsible for most of the oestrus transferred into the room. Heat transfer also occurs through conduction into the room, but much slower. Heat transfer by convection as well occurs through common cold air entering the room around windows and hot air leaving the room past ascension upward the chimney.
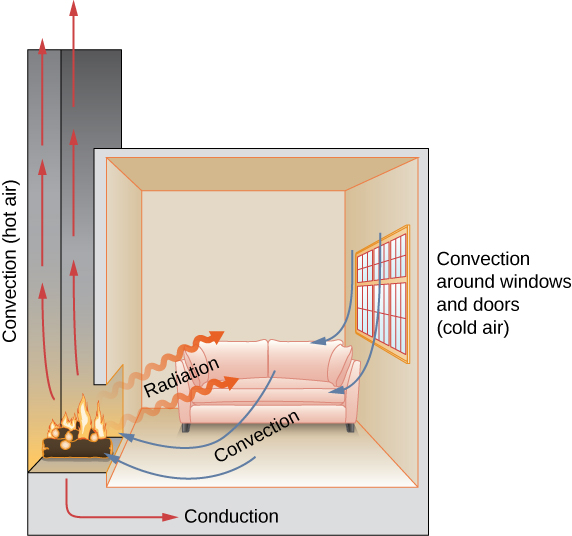
Check Your Understanding Name an example from daily life (dissimilar from the text) for each machinery of heat transfer.
Conduction: Heat transfers into your hands as you hold a hot cup of coffee. Convection: Heat transfers as the barista "steams" cold milk to make hot cocoa. Radiations: Heat transfers from the Sun to a jar of water with tea leaves in it to make "Sun tea." A dandy many other answers are possible.
Conduction
Equally you walk barefoot across the living room carpet in a cold business firm and then step onto the kitchen tile floor, your feet feel colder on the tile. This result is intriguing, since the carpet and tile floor are both at the aforementioned temperature. The different sensation is explained by the dissimilar rates of heat transfer: The heat loss is faster for skin in contact with the tiles than with the carpet, and then the sensation of cold is more than intense.
Some materials conduct thermal free energy faster than others. (Figure) shows a textile that conducts heat slowly—information technology is a good thermal insulator, or poor heat conductor—used to reduce heat flow into and out of a house.
Insulation is used to limit the conduction of heat from the inside to the exterior (in winter) and from the outside to the inside (in summer). (credit: Giles Douglas)

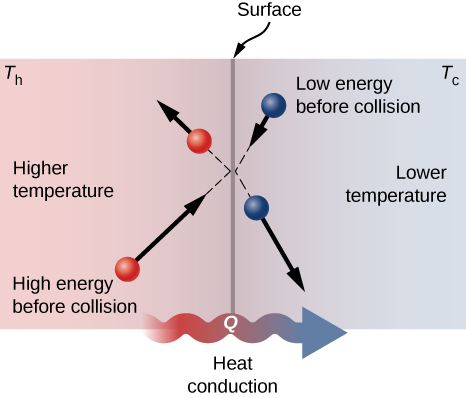
A molecular picture of heat conduction will help justify the equation that describes it. (Figure) shows molecules in two bodies at different temperatures, ![]() and
and ![]() for "hot" and "common cold." The boilerplate kinetic energy of a molecule in the hot body is higher than in the colder trunk. If two molecules collide, energy transfers from the high-energy to the depression-energy molecule. In a metal, the flick would also include free valence electrons colliding with each other and with atoms, likewise transferring free energy. The cumulative effect of all collisions is a net flux of rut from the hotter body to the colder body. Thus, the rate of heat transfer increases with increasing temperature difference
for "hot" and "common cold." The boilerplate kinetic energy of a molecule in the hot body is higher than in the colder trunk. If two molecules collide, energy transfers from the high-energy to the depression-energy molecule. In a metal, the flick would also include free valence electrons colliding with each other and with atoms, likewise transferring free energy. The cumulative effect of all collisions is a net flux of rut from the hotter body to the colder body. Thus, the rate of heat transfer increases with increasing temperature difference ![]() If the temperatures are the aforementioned, the net estrus transfer rate is zero. Because the number of collisions increases with increasing area, heat conduction is proportional to the cantankerous-exclusive area—a second factor in the equation.
If the temperatures are the aforementioned, the net estrus transfer rate is zero. Because the number of collisions increases with increasing area, heat conduction is proportional to the cantankerous-exclusive area—a second factor in the equation.
Molecules in ii bodies at different temperatures have unlike average kinetic energies. Collisions occurring at the contact surface tend to transfer energy from loftier-temperature regions to low-temperature regions. In this analogy, a molecule in the lower-temperature region (correct side) has depression energy before collision, but its energy increases afterward colliding with a high-energy molecule at the contact surface. In contrast, a molecule in the higher-temperature region (left side) has high energy before collision, but its energy decreases afterwards colliding with a depression-energy molecule at the contact surface.
A third quantity that affects the conduction rate is the thickness of the material through which heat transfers. (Figure) shows a slab of material with a higher temperature on the left than on the right. Heat transfers from the left to the correct past a series of molecular collisions. The greater the distance between hot and cold, the more time the fabric takes to transfer the same corporeality of heat.
Heat conduction occurs through any material, represented here past a rectangular bar, whether window glass or walrus blubber.
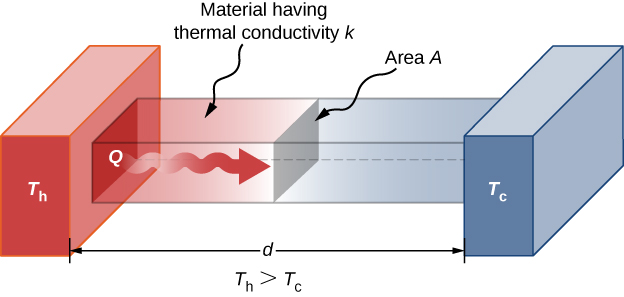
All iv of these quantities appear in a simple equation deduced from and confirmed past experiments. The rate of conductive heat transfer through a slab of cloth, such as the ane in (Figure), is given by
![]()
where P is the power or charge per unit of heat transfer in watts or in kilocalories per second, A and d are its surface area and thickness, as shown in (Effigy), ![]() is the temperature difference across the slab, and yard is the thermal conductivity of the material. (Figure) gives representative values of thermal conductivity.
is the temperature difference across the slab, and yard is the thermal conductivity of the material. (Figure) gives representative values of thermal conductivity.
More generally, nosotros can write
![]()
where x is the coordinate in the direction of rut flow. Since in (Figure), the power and area are constant, dT/dx is constant, and the temperature decreases linearly from ![]() to
to ![]()
| Substance | Thermal Electrical conductivity k |
|---|---|
| Diamond | 2000 |
| Silver | 420 |
| Copper | 390 |
| Aureate | 318 |
| Aluminum | 220 |
| Steel iron | 80 |
| Steel (stainless) | 14 |
| Ice | 2.2 |
| Glass (average) | 0.84 |
| Concrete brick | 0.84 |
| Water | 0.6 |
| Fatty tissue (without blood) | 0.2 |
| Asbestos | 0.16 |
| Plasterboard | 0.16 |
| Wood | 0.08–0.16 |
| Snowfall (dry) | 0.10 |
| Cork | 0.042 |
| Drinking glass wool | 0.042 |
| Wool | 0.04 |
| Down feathers | 0.025 |
| Air | 0.023 |
| Polystyrene foam | 0.010 |
Computing Oestrus Transfer through Conduction A polystyrene foam icebox has a total expanse of ![]() and walls with an boilerplate thickness of ii.50 cm. The box contains ice, water, and canned beverages at
and walls with an boilerplate thickness of ii.50 cm. The box contains ice, water, and canned beverages at ![]() The inside of the box is kept cold past melting water ice. How much ice melts in one day if the icebox is kept in the trunk of a car at
The inside of the box is kept cold past melting water ice. How much ice melts in one day if the icebox is kept in the trunk of a car at ![]() ?
?
Strategy This question involves both heat for a phase change (melting of ice) and the transfer of heat by conduction. To find the amount of ice melted, we must find the net rut transferred. This value tin can be obtained by calculating the rate of rut transfer past conduction and multiplying by time.
Solution Kickoff nosotros place the knowns.
![]() for polystyrene foam;
for polystyrene foam; ![]()
![]() ;
;![]()
![]() ;
; ![]()
Then we identify the unknowns. We need to solve for the mass of the ice, m. We besides need to solve for the net heat transferred to melt the ice, Q. The charge per unit of heat transfer by conduction is given by
![]()
The heat used to melt the ice is ![]() .Nosotros insert the known values:
.Nosotros insert the known values:
![]()
Multiplying the rate of heat transfer by the time nosotros obtain
![]()
We set this equal to the heat transferred to melt the ice, ![]() and solve for the mass m:
and solve for the mass m:
![]()
Significance The result of 3.44 kg, or nigh 7.6 lb, seems about correct, based on experience. Yous might expect to use near a 4 kg (7–10 lb) bag of ice per day. A little extra ice is required if you add whatever warm food or beverages.
(Figure) shows that polystyrene foam is a very poor conductor and thus a proficient insulator. Other skilful insulators include fiberglass, wool, and goosedown feathers. Like polystyrene foam, these all comprise many small pockets of air, taking advantage of air's poor thermal conductivity.
In developing insulation, the smaller the conductivity k and the larger the thickness d, the better. Thus, the ratio d/yard, called the R gene , is large for a skillful insulator. The charge per unit of conductive heat transfer is inversely proportional to R. R factors are most ordinarily quoted for household insulation, refrigerators, and the like. Unfortunately, in the U.s., R is all the same in non-metric units of ![]() , although the unit usually goes unstated [1 British thermal unit of measurement (Btu) is the amount of energy needed to change the temperature of ane.0 lb of water past
, although the unit usually goes unstated [1 British thermal unit of measurement (Btu) is the amount of energy needed to change the temperature of ane.0 lb of water past ![]() , which is 1055.1 J]. A couple of representative values are an R cistron of xi for three.five-inch-thick fiberglass batts (pieces) of insulation and an R factor of 19 for vi.5-inch-thick fiberglass batts ((Figure)). In the US, walls are commonly insulated with three.5-inch batts, whereas ceilings are usually insulated with vi.5-inch batts. In common cold climates, thicker batts may exist used.
, which is 1055.1 J]. A couple of representative values are an R cistron of xi for three.five-inch-thick fiberglass batts (pieces) of insulation and an R factor of 19 for vi.5-inch-thick fiberglass batts ((Figure)). In the US, walls are commonly insulated with three.5-inch batts, whereas ceilings are usually insulated with vi.5-inch batts. In common cold climates, thicker batts may exist used.
The fiberglass batt is used for insulation of walls and ceilings to prevent rut transfer between the inside of the building and the outside surroundings. (credit: Tracey Nicholls)

Note that in (Figure), almost of the best thermal conductors—argent, copper, gold, and aluminum—are also the best electrical conductors, because they contain many costless electrons that can transport thermal energy. (Diamond, an electrical insulator, conducts rut past atomic vibrations.) Cooking utensils are typically made from adept conductors, but the handles of those used on the stove are made from good insulators (bad conductors).
Two Conductors End to End A steel rod and an aluminum rod, each of bore 1.00 cm and length 25.0 cm, are welded terminate to end. One end of the steel rod is placed in a big tank of boiling water at ![]() , while the far end of the aluminum rod is placed in a large tank of h2o at
, while the far end of the aluminum rod is placed in a large tank of h2o at ![]() . The rods are insulated so that no heat escapes from their surfaces. What is the temperature at the joint, and what is the rate of heat conduction through this blended rod?
. The rods are insulated so that no heat escapes from their surfaces. What is the temperature at the joint, and what is the rate of heat conduction through this blended rod?
Strategy The rut that enters the steel rod from the humid h2o has no place to go just through the steel rod, and so through the aluminum rod, to the cold water. Therefore, nosotros tin can equate the rate of conduction through the steel to the rate of conduction through the aluminum.
Nosotros repeat the adding with a 2d method, in which we use the thermal resistance R of the rod, since it simply adds when 2 rods are joined end to end. (We will use a similar method in the chapter on direct-current circuits.)
Solution
- Identify the knowns and catechumen them to SI units.
The length of each rod is the cross-exclusive area of each rod is
the cross-exclusive area of each rod is 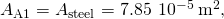 the thermal conductivity of aluminum is
the thermal conductivity of aluminum is 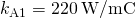 , the thermal electrical conductivity of steel is
, the thermal electrical conductivity of steel is 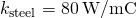 , the temperature at the hot cease is
, the temperature at the hot cease is 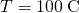 , and the temperature at the common cold end is
, and the temperature at the common cold end is 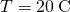 .
. - Calculate the heat-conduction rate through the steel rod and the heat-conduction charge per unit through the aluminum rod in terms of the unknown temperature T at the articulation:
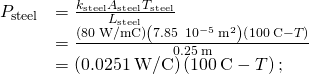
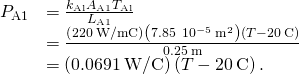
- Fix the ii rates equal and solve for the unknown temperature:
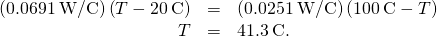
- Calculate either rate:
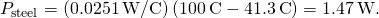
- If desired, check your answer by calculating the other charge per unit.
Solution
- Remember that
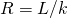 . Now
. Now 
- We know that
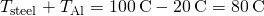 . We also know that
. We also know that 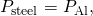 and nosotros denote that rate of oestrus menses by P. Combine the equations:
and nosotros denote that rate of oestrus menses by P. Combine the equations:
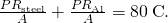
Thus, we can just add R factors. Now,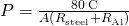 .
. - Find the
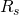 from the known quantities:
from the known quantities:
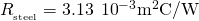
and
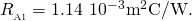
- Substitute these values in to find
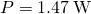 as earlier.
as earlier. - Determine
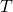 for the aluminum rod (or for the steel rod) and use it to find T at the joint.
for the aluminum rod (or for the steel rod) and use it to find T at the joint.
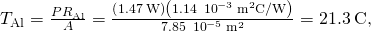
so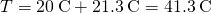 , as in Solution
, as in Solution 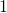 .
. - If desired, check by determining
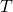 for the other rod.
for the other rod.
Significance In practice, adding R values is mutual, as in calculating the R value of an insulated wall. In the analogous situation in electronics, the resistance corresponds to AR in this problem and is condiment even when the areas are diff, as is common in electronics. Our equation for estrus conduction can exist used only when the areas are equal; otherwise, we would have a problem in iii-dimensional heat menses, which is beyond our telescopic.
Check Your Understanding How does the charge per unit of heat transfer by conduction change when all spatial dimensions are doubled?
Considering area is the product of two spatial dimensions, it increases past a factor of four when each dimension is doubled ![]() . The distance, yet, simply doubles. Because the temperature difference and the coefficient of thermal conductivity are contained of the spatial dimensions, the charge per unit of heat transfer past conduction increases by a factor of four divided past two, or two:
. The distance, yet, simply doubles. Because the temperature difference and the coefficient of thermal conductivity are contained of the spatial dimensions, the charge per unit of heat transfer past conduction increases by a factor of four divided past two, or two:
![]() .
.
Conduction is caused by the random motion of atoms and molecules. As such, it is an ineffective mechanism for heat transport over macroscopic distances and short times. For example, the temperature on Earth would be unbearably cold during the dark and extremely hot during the day if heat transport in the temper were only through conduction. Also, machine engines would overheat unless at that place was a more efficient manner to remove backlog rut from the pistons. The next module discusses the of import heat-transfer machinery in such situations.
Convection
In convection, thermal energy is carried by the big-scale flow of matter. Information technology can be divided into two types. In forced convection , the flow is driven past fans, pumps, and the like. A simple example is a fan that blows air past you lot in hot surroundings and cools you by replacing the air heated by your body with cooler air. A more complicated example is the cooling arrangement of a typical motorcar, in which a pump moves coolant through the radiator and engine to cool the engine and a fan blows air to cool the radiator.
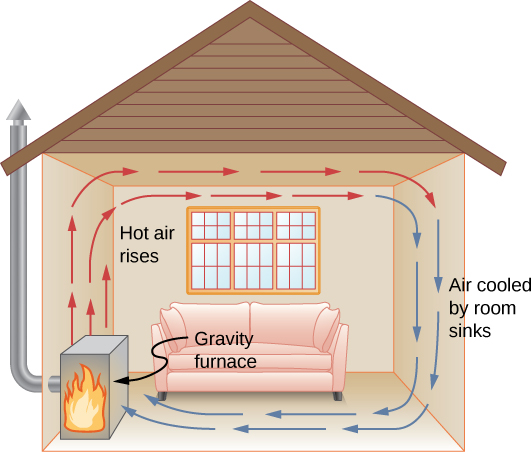
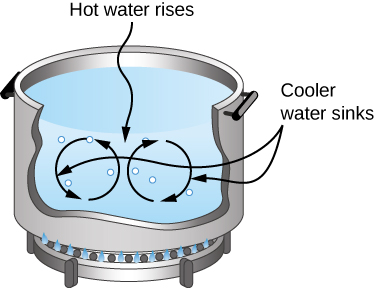
In free or natural convection , the menses is driven by buoyant forces: hot fluid rises and cold fluid sinks because density decreases as temperature increases. The firm in (Figure) is kept warm by natural convection, as is the pot of water on the stove in (Figure). Sea currents and large-scale atmospheric circulation, which event from the buoyancy of warm air and water, transfer hot air from the tropics toward the poles and cold air from the poles toward the tropics. (Earth'due south rotation interacts with those flows, causing the observed east flow of air in the temperate zones.)
Air heated by a so-called gravity furnace expands and rises, forming a convective loop that transfers energy to other parts of the room. As the air is cooled at the ceiling and outside walls, information technology contracts, somewhen becoming denser than room air and sinking to the flooring. A properly designed heating organization using natural convection, like this one, can heat a home quite efficiently.
Natural convection plays an of import function in heat transfer inside this pot of water. Once conducted to the inside, oestrus transfer to other parts of the pot is mostly by convection. The hotter water expands, decreases in density, and rises to transfer estrus to other regions of the water, while colder water sinks to the bottom. This process keeps repeating.
Natural convection like that of (Figure) and (Effigy), simply acting on rock in Earth's mantle, drives plate tectonics that are the motions that have shaped Earth'due south surface.
Convection is ordinarily more than complicated than conduction. Across noting that the convection charge per unit is frequently approximately proportional to the temperature difference, nosotros will not practise any quantitative work comparable to the formula for conduction. Even so, we tin can describe convection qualitatively and relate convection rates to heat and time. Air is a poor conductor, so convection dominates rut transfer past air. Therefore, the corporeality of available infinite for airflow determines whether air transfers estrus quickly or slowly. There is little estrus transfer in a space filled with air with a small amount of other material that prevents flow. The space betwixt the inside and exterior walls of a typical American house, for case, is about 9 cm (iii.5 in.)—big plenty for convection to piece of work effectively. The addition of wall insulation prevents airflow, then rut loss (or gain) is decreased. On the other paw, the gap between the ii panes of a double-paned window is about 1 cm, which largely prevents convection and takes advantage of air'south depression electrical conductivity reduce rut loss. Fur, cloth, and fiberglass as well take advantage of the low electrical conductivity of air by trapping it in spaces too pocket-size to support convection ((Figure)).
Fur is filled with air, breaking it upwardly into many small pockets. Convection is very dull here, because the loops are so minor. The low conductivity of air makes fur a very proficient lightweight insulator.
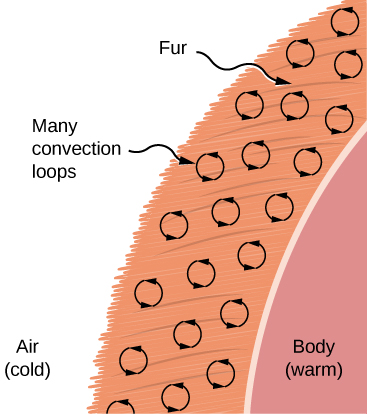
Some interesting phenomena happen when convection is accompanied by a phase modify. The combination allows u.s. to absurd off past sweating even if the temperature of the surrounding air exceeds body temperature. Heat from the pare is required for sweat to evaporate from the pare, but without air flow, the air becomes saturated and evaporation stops. Air flow caused by convection replaces the saturated air past dry air and evaporation continues.
Calculating the Flow of Mass during Convection The average person produces heat at the rate of nearly 120 W when at rest. At what rate must water evaporate from the body to become rid of all this energy? (For simplicity, we assume this evaporation occurs when a person is sitting in the shade and surrounding temperatures are the same as peel temperature, eliminating rut transfer by other methods.)
Strategy Free energy is needed for this phase change (![]() ). Thus, the energy loss per unit time is
). Thus, the energy loss per unit time is
![]()
Nosotros divide both sides of the equation by ![]() to observe that the mass evaporated per unit time is
to observe that the mass evaporated per unit time is
![]()
Solution Insert the value of the latent rut from (Figure), ![]() . This yields
. This yields
![]()
Significance Evaporating well-nigh 3 g/min seems reasonable. This would be about 180 chiliad (well-nigh 7 oz.) per hour. If the air is very dry out, the sweat may evaporate without even being noticed. A significant amount of evaporation also takes place in the lungs and animate passages.
Another important case of the combination of phase change and convection occurs when water evaporates from the oceans. Heat is removed from the ocean when water evaporates. If the water vapor condenses in liquid aerosol equally clouds form, possibly far from the body of water, estrus is released in the atmosphere. Thus, there is an overall transfer of heat from the ocean to the temper. This process is the driving ability behind thunderheads, those not bad cumulus clouds that ascent as much as 20.0 km into the stratosphere ((Figure)). H2o vapor carried in by convection condenses, releasing tremendous amounts of energy. This energy causes the air to expand and rise to colder altitudes. More condensation occurs in these regions, which in turn drives the cloud even higher. This machinery is an case of positive feedback, since the process reinforces and accelerates itself. It sometimes produces violent storms, with lightning and hail. The same mechanism drives hurricanes.
This time-lapse video shows convection currents in a thunderstorm, including "rolling" motion like to that of boiling h2o.
Cumulus clouds are acquired by water vapor that rises because of convection. The ascent of clouds is driven by a positive feedback mechanism. (credit: "Amada44"/Wikimedia Eatables)

Check Your Agreement Explain why using a fan in the summer feels refreshing.
Using a fan increases the flow of air: Warm air near your body is replaced by cooler air from elsewhere. Convection increases the rate of heat transfer and then that moving air "feels" cooler than still air.
Radiation
You can feel the heat transfer from the Sunday. The space between Globe and the Sun is largely empty, so the Sun warms u.s.a. without any possibility of estrus transfer by convection or conduction. Similarly, you can sometimes tell that the oven is hot without touching its door or looking inside—it may just warm you equally yous walk past. In these examples, rut is transferred by radiation ((Figure)). That is, the hot body emits electromagnetic waves that are absorbed by the peel. No medium is required for electromagnetic waves to propagate. Unlike names are used for electromagnetic waves of different wavelengths: radio waves, microwaves, infrared radiation, visible calorie-free, ultraviolet radiation, 10-rays, and gamma rays.
Most of the estrus transfer from this fire to the observers occurs through infrared radiation. The visible light, although dramatic, transfers relatively little thermal energy. Convection transfers energy away from the observers as hot air rises, while conduction is negligibly slow here. Skin is very sensitive to infrared radiation, then you can sense the presence of a burn without looking at information technology straight. (credit: Daniel O'Neil)

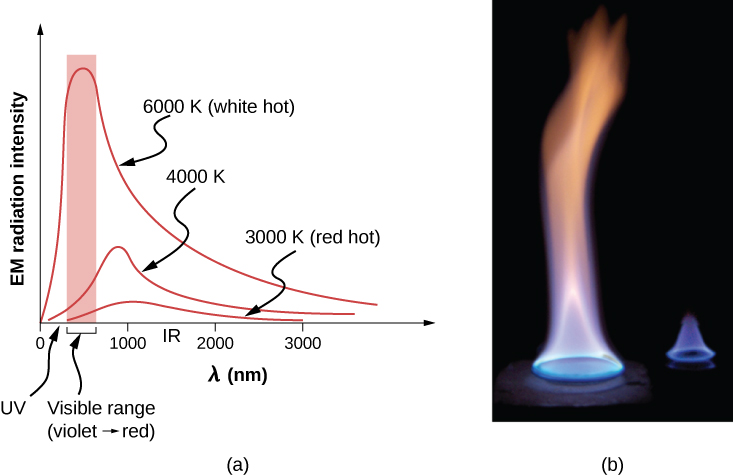
The energy of electromagnetic radiation varies over a wide range, depending on the wavelength: A shorter wavelength (or higher frequency) corresponds to a college free energy. Because more heat is radiated at higher temperatures, college temperatures produce more intensity at every wavelength but peculiarly at shorter wavelengths. In visible light, wavelength determines color—red has the longest wavelength and violet the shortest—so a temperature change is accompanied by a color change. For example, an electrical heating element on a stove glows from red to orange, while the college-temperature steel in a blast furnace glows from xanthous to white. Infrared radiation is the predominant form radiated by objects cooler than the electric element and the steel. The radiated energy every bit a office of wavelength depends on its intensity, which is represented in (Figure) by the height of the distribution. (Electromagnetic Waves explains more about the electromagnetic spectrum, and Photons and Matter Waves discusses why the decrease in wavelength corresponds to an increase in energy.)
(a) A graph of the spectrum of electromagnetic waves emitted from an ideal radiator at iii different temperatures. The intensity or rate of radiation emission increases dramatically with temperature, and the spectrum shifts downwardly in wavelength toward the visible and ultraviolet parts of the spectrum. The shaded portion denotes the visible function of the spectrum. It is apparent that the shift toward the ultraviolet with temperature makes the visible advent shift from red to white to blueish as temperature increases. (b) Note the variations in colour corresponding to variations in flame temperature.
The charge per unit of heat transfer by radiation also depends on the object's color. Black is the about effective, and white is the to the lowest degree constructive. On a clear summertime twenty-four hours, blackness asphalt in a parking lot is hotter than adjacent gray sidewalk, because black absorbs better than grey ((Figure)). The reverse is also true—black radiates improve than grey. Thus, on a clear summertime dark, the asphalt is colder than the gray sidewalk, considering black radiates the free energy more than rapidly than gray. A perfectly blackness object would be an ideal radiator and an ideal absorber, as it would capture all the radiation that falls on information technology. In contrast, a perfectly white object or a perfect mirror would reflect all radiation, and a perfectly transparent object would transmit it all ((Figure)). Such objects would not emit whatsoever radiation. Mathematically, the color is represented by the emissivity e. A "blackbody" radiator would have an ![]() , whereas a perfect reflector or transmitter would have
, whereas a perfect reflector or transmitter would have ![]() . For real examples, tungsten light bulb filaments have an e of near 0.5, and carbon black (a material used in printer toner) has an emissivity of about 0.95.
. For real examples, tungsten light bulb filaments have an e of near 0.5, and carbon black (a material used in printer toner) has an emissivity of about 0.95.
The darker pavement is hotter than the lighter pavement (much more of the ice on the right has melted), although both have been in the sunlight for the same time. The thermal conductivities of the pavements are the same.

A black object is a good absorber and a good radiator, whereas a white, clear, or silver object is a poor absorber and a poor radiator.
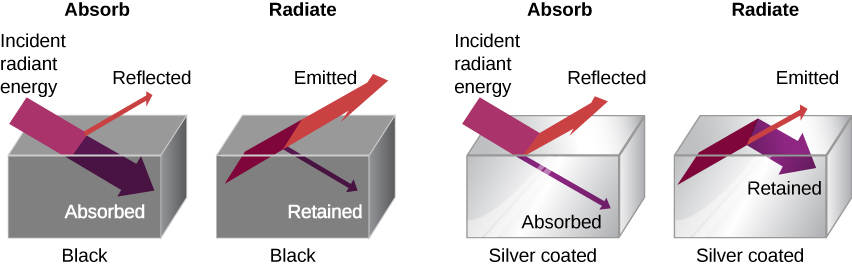
To see that, consider a silver object and a blackness object that can exchange rut past radiation and are in thermal equilibrium. We know from feel that they will stay in equilibrium (the outcome of a principle that will be discussed at length in 2nd Law of Thermodynamics). For the black object's temperature to stay constant, it must emit as much radiation as information technology absorbs, and then it must exist equally good at radiating as absorbing. Like considerations prove that the argent object must radiate as niggling as information technology absorbs. Thus, i property, emissivity, controls both radiation and absorption.
Finally, the radiated heat is proportional to the object'due south surface area, since every part of the surface radiates. If you knock apart the dress-down of a burn, the radiation increases noticeably due to an increase in radiating surface area.
The rate of heat transfer past emitted radiation is described past the Stefan-Boltzmann police of radiation:
![]()
where ![]() is the Stefan-Boltzmann constant, a combination of fundamental constants of nature; A is the surface area of the object; and T is its temperature in kelvins.
is the Stefan-Boltzmann constant, a combination of fundamental constants of nature; A is the surface area of the object; and T is its temperature in kelvins.
The proportionality to the 4th power of the absolute temperature is a remarkably strong temperature dependence. It allows the detection of fifty-fifty small temperature variations. Images called thermographs tin can exist used medically to observe regions of abnormally high temperature in the body, perhaps indicative of disease. Like techniques can exist used to detect estrus leaks in homes ((Figure)), optimize operation of nail furnaces, ameliorate comfort levels in work environments, and even remotely map Earth's temperature contour.
A thermograph of office of a building shows temperature variations, indicating where rut transfer to the exterior is nearly severe. Windows are a major region of heat transfer to the outside of homes. (credit: US Army)

The Stefan-Boltzmann equation needs simply slight refinement to bargain with a uncomplicated case of an object's assimilation of radiation from its environs. Assuming that an object with a temperature ![]() is surrounded by an environs with uniform temperature
is surrounded by an environs with uniform temperature ![]() , the net rate of heat transfer by radiation is
, the net rate of heat transfer by radiation is
![]()
where e is the emissivity of the object alone. In other words, it does not matter whether the surroundings are white, grey, or black: The rest of radiation into and out of the object depends on how well it emits and absorbs radiation. When ![]() the quantity
the quantity ![]() is positive, that is, the net oestrus transfer is from hot to cold.
is positive, that is, the net oestrus transfer is from hot to cold.
Before doing an example, we have a complication to discuss: different emissivities at unlike wavelengths. If the fraction of incident radiation an object reflects is the same at all visible wavelengths, the object is grey; if the fraction depends on the wavelength, the object has some other colour. For instance, a cherry or reddish object reflects cherry-red lite more strongly than other visible wavelengths. Considering information technology absorbs less red, it radiates less scarlet when hot. Differential reflection and absorption of wavelengths outside the visible range have no outcome on what we come across, but they may have physically important effects. Skin is a very good absorber and emitter of infrared radiations, having an emissivity of 0.97 in the infrared spectrum. Thus, in spite of the obvious variations in skin color, we are all most black in the infrared. This high infrared emissivity is why nosotros can and then easily feel radiation on our peel. It is also the basis for the effectiveness of night-vision scopes used by law enforcement and the military to notice human beings.
Calculating the Net Heat Transfer of a Person What is the charge per unit of heat transfer by radiations of an unclothed person standing in a dark room whose ambient temperature is ![]() ? The person has a normal skin temperature of
? The person has a normal skin temperature of ![]() and a surface area of
and a surface area of ![]() The emissivity of skin is 0.97 in the infrared, the part of the spectrum where the radiation takes identify.
The emissivity of skin is 0.97 in the infrared, the part of the spectrum where the radiation takes identify.
Strategy Nosotros can solve this by using the equation for the rate of radiative heat transfer.
Solution Insert the temperature values ![]() and
and ![]() , and then that
, and then that
![Rendered by QuickLaTeX.com \begin{array}{cc}\hfill \frac{Q}{t}& =\sigma eA\left({T}_{2}{}^{4}-{T}_{1}{}^{4}\right)\hfill \\ & =\left(5.67\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-8}\phantom{\rule{0.2em}{0ex}}\text{J/s}·{\text{m}}^{2}·{\text{K}}^{4}\right)\left(0.97\right)\left(1.50\phantom{\rule{0.2em}{0ex}}{\text{m}}^{2}\right)\left[{\left(295\phantom{\rule{0.2em}{0ex}}\text{K}\right)}^{4}-{\left(306\phantom{\rule{0.2em}{0ex}}\text{K}\right)}^{4}\right]\hfill \\ & =-99\phantom{\rule{0.2em}{0ex}}\text{J/s}=-99\phantom{\rule{0.2em}{0ex}}\text{W}\text{.}\hfill \end{array}](https://opentextbc.ca/universityphysicsv2openstax/wp-content/ql-cache/quicklatex.com-6c71c15f7eaf427086b7090a6cf2ceb6_l3.png)
Significance This value is a significant rate of estrus transfer to the surroundings (note the minus sign), considering that a person at rest may produce energy at the rate of 125 W and that conduction and convection are also transferring energy to the environment. Indeed, we would probably expect this person to feel cold. Article of clothing significantly reduces heat transfer to the environment past all mechanisms, because clothing slows downwardly both conduction and convection, and has a lower emissivity (peculiarly if information technology is low-cal-colored) than skin.
The average temperature of Earth is the subject of much electric current discussion. Earth is in radiative contact with both the Sun and dark space, so we cannot use the equation for an environment at a compatible temperature. Earth receives almost all its energy from radiation of the Lord's day and reflects some of it back into outer infinite. Conversely, dark space is very cold, about 3 Thousand, and then that Earth radiates energy into the dark heaven. The rate of heat transfer from soil and grasses can be so rapid that frost may occur on articulate summer evenings, even in warm latitudes.
The average temperature of Earth is adamant by its energy residue. To a get-go approximation, it is the temperature at which Earth radiates heat to space as fast as it receives free energy from the Sun.
An important parameter in computing the temperature of Earth is its emissivity (e). On average, it is about 0.65, but calculation of this value is complicated by the corking mean solar day-to-day variation in the highly reflective cloud coverage. Considering clouds have lower emissivity than either oceans or country masses, they reverberate some of the radiations dorsum to the surface, greatly reducing heat transfer into dark space, merely equally they greatly reduce rut transfer into the atmosphere during the day. At that place is negative feedback (in which a alter produces an effect that opposes that change) between clouds and oestrus transfer; higher temperatures evaporate more h2o to form more than clouds, which reverberate more than radiation back into space, reducing the temperature.
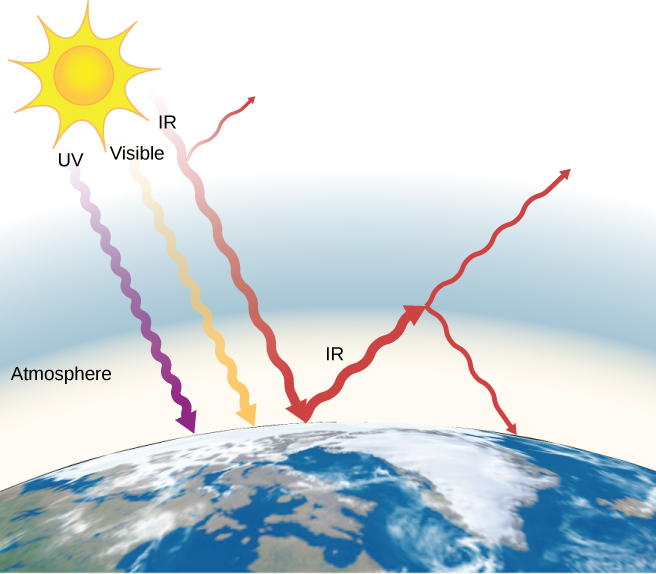
The often-mentioned greenhouse effect is directly related to the variation of Earth's emissivity with wavelength ((Figure)). The greenhouse effect is a natural phenomenon responsible for providing temperatures suitable for life on Globe and for making Venus unsuitable for human being life. Most of the infrared radiation emitted from Earth is captivated past carbon dioxide (![]() ) and h2o (
) and h2o (![]() ) in the atmosphere and then re-radiated into outer space or back to Earth. Re-radiation dorsum to World maintains its surface temperature about
) in the atmosphere and then re-radiated into outer space or back to Earth. Re-radiation dorsum to World maintains its surface temperature about ![]() higher than information technology would be if there were no atmosphere. (The glass walls and roof of a greenhouse increase the temperature inside past blocking convective estrus losses, not radiative losses.)
higher than information technology would be if there were no atmosphere. (The glass walls and roof of a greenhouse increase the temperature inside past blocking convective estrus losses, not radiative losses.)
The greenhouse issue is the name given to the increase of Earth's temperature due to absorption of radiation in the temper. The atmosphere is transparent to incoming visible radiation and most of the Sun's infrared. The Earth absorbs that free energy and re-emits it. Since Earth's temperature is much lower than the Sun's, it re-emits the energy at much longer wavelengths, in the infrared. The atmosphere absorbs much of that infrared radiations and radiates about one-half of the energy back downwards, keeping Earth warmer than it would otherwise be. The amount of trapping depends on concentrations of trace gases such equally carbon dioxide, and an increase in the concentration of these gases increases Earth's surface temperature.
The greenhouse result is central to the word of global warming due to emission of carbon dioxide and methane (and other greenhouse gases) into Globe'due south atmosphere from industry, transportation, and farming. Changes in global climate could lead to more than intense storms, precipitation changes (affecting agriculture), reduction in rain wood biodiversity, and ascent sea levels.
Yous can explore a simulation of the greenhouse effect that takes the betoken of view that the atmosphere scatters (redirects) infrared radiation rather than absorbing information technology and reradiating information technology. You lot may desire to run the simulation first with no greenhouse gases in the temper and then wait at how adding greenhouse gases affects the infrared radiations from the Earth and the Globe'south temperature.
Check Your Understanding How much greater is the charge per unit of heat radiation when a body is at the temperature ![]() than when it is at the temperature
than when it is at the temperature ![]() ?
?
The radiated heat is proportional to the fourth ability of the absolute temperature. Considering ![]() and
and ![]() , the charge per unit of heat transfer increases by nearly 30% of the original rate.
, the charge per unit of heat transfer increases by nearly 30% of the original rate.
Summary
- Heat is transferred by three unlike methods: conduction, convection, and radiation.
- Heat conduction is the transfer of estrus between two objects in direct contact with each other.
- The rate of heat transfer P (energy per unit time) is proportional to the temperature difference
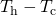 and the contact expanse A and inversely proportional to the distance d between the objects.
and the contact expanse A and inversely proportional to the distance d between the objects. - Convection is oestrus transfer by the macroscopic movement of mass. Convection tin be natural or forced, and mostly transfers thermal free energy faster than conduction. Convection that occurs forth with a phase change can transfer energy from cold regions to warm ones.
- Radiations is estrus transfer through the emission or absorption of electromagnetic waves.
- The rate of radiative heat transfer is proportional to the emissivity east. For a perfect blackbody,
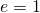 , whereas a perfectly white, articulate, or reflective body has
, whereas a perfectly white, articulate, or reflective body has 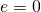 , with real objects having values of e between 1 and 0.
, with real objects having values of e between 1 and 0. - The charge per unit of heat transfer depends on the area and the 4th power of the absolute temperature:
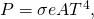
where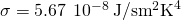 is the Stefan-Boltzmann abiding and e is the emissivity of the body. The net charge per unit of heat transfer from an object past radiations is
is the Stefan-Boltzmann abiding and e is the emissivity of the body. The net charge per unit of heat transfer from an object past radiations is
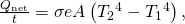
where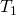 is the temperature of the object surrounded by an environs with uniform temperature
is the temperature of the object surrounded by an environs with uniform temperature 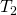 and due east is the emissivity of the object.
and due east is the emissivity of the object.
Conceptual Questions
What are the primary methods of heat transfer from the hot core of Earth to its surface? From Earth's surface to outer infinite?
When our bodies go too warm, they answer by sweating and increasing claret circulation to the surface to transfer thermal energy away from the cadre. What effect will those processes take on a person in a ![]() hot tub?
hot tub?
Increasing apportionment to the surface volition warm the person, as the temperature of the water is warmer than human being body temperature. Sweating volition cause no evaporative cooling under water or in the boiling air immediately above the tub.
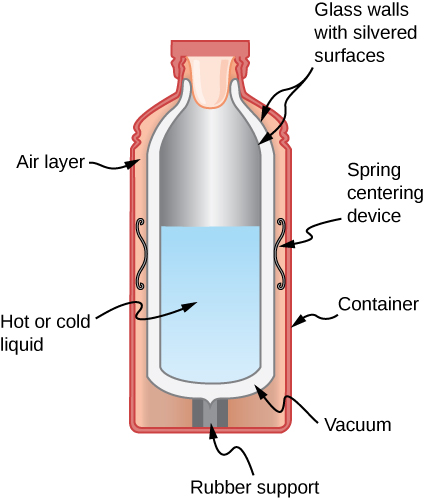
Shown below is a cut-away cartoon of a thermos bottle (besides known as a Dewar flask), which is a device designed specifically to slow down all forms of oestrus transfer. Explain the functions of the various parts, such every bit the vacuum, the silvering of the walls, the sparse-walled long drinking glass neck, the rubber back up, the air layer, and the stopper.
Some electric stoves have a flat ceramic surface with heating elements hidden beneath. A pot placed over a heating element volition be heated, while the surface only a few centimeters abroad is safe to touch. Why is ceramic, with a conductivity less than that of a metal simply greater than that of a proficient insulator, an ideal choice for the stove top?
It spread the rut over the area above the heating elements, evening the temperature there, but does not spread the heat much beyond the heating elements.
Loose-fitting white clothing covering nearly of the body, shown below, is platonic for desert dwellers, both in the hot Sun and during cold evenings. Explicate how such clothing is advantageous during both twenty-four hours and night.

I way to make a fireplace more free energy-efficient is to have room air circulate effectually the outside of the fire box and dorsum into the room. Detail the methods of heat transfer involved.
Heat is conducted from the fire through the burn box to the circulating air and then convected past the air into the room (forced convection).
On cold, articulate nights horses will sleep under the comprehend of large trees. How does this help them keep warm?
When watching a circus during the day in a large, dark-colored tent, yous sense significant heat transfer from the tent. Explain why this occurs.
The tent is heated past the Dominicus and transfers heat to you lot by all iii processes, peculiarly radiations.
Satellites designed to observe the radiation from cold (3 K) dark space take sensors that are shaded from the Sunday, World, and the Moon and are cooled to very low temperatures. Why must the sensors exist at low temperature?
Why are thermometers that are used in atmospheric condition stations shielded from the sunshine? What does a thermometer measure if it is shielded from the sunshine? What does it measure if it is not?
If shielded, it measures the air temperature. If not, information technology measures the combined effect of air temperature and cyberspace radiative estrus gain from the Sun.
Putting a chapeau on a boiling pot greatly reduces the heat transfer necessary to keep it boiling. Explain why.
Your house will exist empty for a while in cold weather, and you desire to save free energy and money. Should you plough the thermostat down to the lowest level that will protect the house from harm such every bit freezing pipes, or leave it at the normal temperature? (If yous don't like coming back to a cold house, imagine that a timer controls the heating system so the house will be warm when you get back.) Explicate your respond.
Turn the thermostat downwards. To take the business firm at the normal temperature, the heating system must supersede all the heat that was lost. For all 3 mechanisms of oestrus transfer, the greater the temperature departure between inside and outside, the more heat is lost and must be replaced. So the house should exist at the everyman temperature that does not let freezing damage.
You cascade coffee into an unlidded loving cup, intending to potable it 5 minutes later. You tin add cream when you cascade the loving cup or right before you beverage it. (The cream is at the same temperature either way. Assume that the cream and coffee come into thermal equilibrium with each other very quickly.) Which way will give you hotter java? What feature of this question is different from the previous one?
Broiling is a method of cooking past radiation, which produces somewhat unlike results from cooking by conduction or convection. A gas flame or electric heating element produces a very high temperature close to the nutrient and above it. Why is radiation the dominant heat-transfer method in this situation?
Air is a practiced insulator, and so there is footling conduction, and the heated air rises, and then at that place is little convection downwards.
On a cold winter morning, why does the metal of a bike feel colder than the wood of a porch?
Issues
(a) Calculate the rate of heat conduction through house walls that are xiii.0 cm thick and have an average thermal conductivity twice that of drinking glass wool. Presume there are no windows or doors. The walls' surface area is ![]() and their inside surface is at
and their inside surface is at ![]() , while their outside surface is at
, while their outside surface is at ![]() . (b) How many 1-kW room heaters would be needed to residual the oestrus transfer due to conduction?
. (b) How many 1-kW room heaters would be needed to residual the oestrus transfer due to conduction?
a. ![]() ; b. One 1-kilowatt room heater is needed.
; b. One 1-kilowatt room heater is needed.
Calculate the rate of heat conduction out of the human body, bold that the core internal temperature is ![]() , the pare temperature is
, the pare temperature is ![]() , the thickness of the fatty tissues between the core and the pare averages one.00 cm, and the surface area is
, the thickness of the fatty tissues between the core and the pare averages one.00 cm, and the surface area is ![]() .
.
84.0 W
A human being consumes 3000 kcal of nutrient in one day, converting most of it to thermal free energy to maintain body temperature. If he loses half this free energy by evaporating h2o (through animate and sweating), how many kilograms of water evaporate?
2.59 kg
(a) What is the charge per unit of heat conduction through the 3.00-cm-thick fur of a large animal having a ![]() area? Assume that the fauna'southward pare temperature is
area? Assume that the fauna'southward pare temperature is ![]() , that the air temperature is
, that the air temperature is ![]() , and that fur has the same thermal conductivity as air. (b) What nutrient intake will the brute need in one solar day to replace this heat transfer?
, and that fur has the same thermal conductivity as air. (b) What nutrient intake will the brute need in one solar day to replace this heat transfer?
a. 39.7 West; b. 820 kcal
Compare the rate of heat conduction through a thirteen.0-cm-thick wall that has an area of ![]() and a thermal conductivity twice that of drinking glass wool with the rate of estrus conduction through a 0.750-cm-thick window that has an area of
and a thermal conductivity twice that of drinking glass wool with the rate of estrus conduction through a 0.750-cm-thick window that has an area of ![]() , assuming the same temperature difference across each.
, assuming the same temperature difference across each.
![]() , so that
, so that
![]()
This gives 0.0288 wall: window, or 35:1 window: wall
Suppose a person is covered head to foot by wool clothing with boilerplate thickness of 2.00 cm and is transferring energy past conduction through the clothing at the charge per unit of 50.0 W. What is the temperature deviation across the clothing, given the expanse is ![]() ?
?
Some stove tops are smooth ceramic for easy cleaning. If the ceramic is 0.600 cm thick and heat conduction occurs through the same area and at the same rate as computed in (Effigy), what is the temperature difference across it? Ceramic has the same thermal conductivity as glass and brick.
One easy way to reduce heating (and cooling) costs is to add together extra insulation in the attic of a firm. Suppose a single-story cubical house already had 15 cm of fiberglass insulation in the attic and in all the exterior surfaces. If y'all added an actress eight.0 cm of fiberglass to the cranium, by what percent would the heating cost of the house drop? Take the house to take dimensions 10 m by 15 one thousand by three.0 m. Ignore air infiltration and estrus loss through windows and doors, and assume that the interior is uniformly at one temperature and the exterior is uniformly at another.
Additional Problems
In 1701, the Danish astronomer Ole Rømer proposed a temperature scale with two fixed points, freezing water at 7.5 degrees, and humid h2o at 60.0 degrees. What is the boiling point of oxygen, 90.2 K, on the Rømer scale?
What is the pct mistake of thinking the melting point of tungsten is ![]() instead of the correct value of 3695 Thou?
instead of the correct value of 3695 Thou?
![]()
An engineer wants to design a structure in which the difference in length between a steel beam and an aluminum beam remains at 0.500 chiliad regardless of temperature, for ordinary temperatures. What must the lengths of the beams be?
How much stress is created in a steel beam if its temperature changes from ![]() to
to ![]() but it cannot expand? For steel, the Young's modulus
but it cannot expand? For steel, the Young's modulus ![]() from Stress, Strain, and Rubberband Modulus. (Ignore the change in area resulting from the expansion.)
from Stress, Strain, and Rubberband Modulus. (Ignore the change in area resulting from the expansion.)
![]() .
.
A brass rod ![]() with a diameter of 0.800 cm and a length of one.20 m when the temperature is
with a diameter of 0.800 cm and a length of one.20 m when the temperature is ![]() , is fixed at both ends. At what temperature is the force in information technology at 36,000 Due north?
, is fixed at both ends. At what temperature is the force in information technology at 36,000 Due north?
A mercury thermometer nonetheless in utilise for meteorology has a bulb with a volume of ![]() and a tube for the mercury to expand into of inside diameter 0.130 mm. (a) Neglecting the thermal expansion of the glass, what is the spacing between marks
and a tube for the mercury to expand into of inside diameter 0.130 mm. (a) Neglecting the thermal expansion of the glass, what is the spacing between marks ![]() autonomously? (b) If the thermometer is made of ordinary glass (not a skillful idea), what is the spacing?
autonomously? (b) If the thermometer is made of ordinary glass (not a skillful idea), what is the spacing?
a. ![]() cm; b.
cm; b. ![]() cm
cm
Some gun fanciers make their own bullets, which involves melting atomic number 82 and casting it into lead slugs. How much estrus transfer is needed to enhance the temperature and cook 0.500 kg of lead, starting from ![]() ?
?
A 0.800-kg iron cylinder at a temperature of ![]() is dropped into an insulated breast of 1.00 kg of ice at its melting point. What is the last temperature, and how much water ice has melted?
is dropped into an insulated breast of 1.00 kg of ice at its melting point. What is the last temperature, and how much water ice has melted?
![]() . All of the ice melted.
. All of the ice melted.
Repeat the preceding trouble with ii.00 kg of water ice instead of ane.00 kg.
Repeat the preceding trouble with 0.500 kg of ice, assuming that the water ice is initially in a copper container of mass 1.50 kg in equilibrium with the ice.
![]() , all the water ice melted
, all the water ice melted
A thirty.0-1000 ice cube at its melting betoken is dropped into an aluminum calorimeter of mass 100.0 thousand in equilibrium at ![]() with 300.0 g of an unknown liquid. The final temperature is
with 300.0 g of an unknown liquid. The final temperature is ![]() . What is the heat capacity of the liquid?
. What is the heat capacity of the liquid?
(a) Calculate the rate of heat conduction through a double-paned window that has a ![]() area and is made of two panes of 0.800-cm-thick glass separated by a i.00-cm air gap. The inside surface temperature is
area and is made of two panes of 0.800-cm-thick glass separated by a i.00-cm air gap. The inside surface temperature is ![]() while that on the outside is
while that on the outside is ![]() (Hint: There are identical temperature drops beyond the two glass panes. First find these so the temperature drop beyond the air gap. This problem ignores the increased heat transfer in the air gap due to convection.) (b) Calculate the rate of heat conduction through a 1.60-cm-thick window of the same area and with the aforementioned temperatures. Compare your answer with that for office (a).
(Hint: There are identical temperature drops beyond the two glass panes. First find these so the temperature drop beyond the air gap. This problem ignores the increased heat transfer in the air gap due to convection.) (b) Calculate the rate of heat conduction through a 1.60-cm-thick window of the same area and with the aforementioned temperatures. Compare your answer with that for office (a).
a. 83 W; b. ![]() ; The single-pane window has a rate of heat conduction equal to 1969/83, or 24 times that of a double-pane window.
; The single-pane window has a rate of heat conduction equal to 1969/83, or 24 times that of a double-pane window.
(a) An exterior wall of a house is 3 m tall and 10 m wide. It consists of a layer of drywall with an R factor of 0.56, a layer iii.v inches thick filled with fiberglass batts, and a layer of insulated siding with an R gene of ii.half dozen. The wall is built so well that there are no leaks of air through it. When the within of the wall is at ![]() and the exterior is at
and the exterior is at ![]() , what is the rate of oestrus flow through the wall? (b) More realistically, the 3.5-inch infinite as well contains two-past-4 studs—wooden boards ane.5 inches past iii.5 inches oriented and so that three.five-inch dimension extends from the drywall to the siding. They are "on 16-inch centers," that is, the centers of the studs are 16 inches apart. What is the rut current in this state of affairs? Don't worry virtually one stud more or less.
, what is the rate of oestrus flow through the wall? (b) More realistically, the 3.5-inch infinite as well contains two-past-4 studs—wooden boards ane.5 inches past iii.5 inches oriented and so that three.five-inch dimension extends from the drywall to the siding. They are "on 16-inch centers," that is, the centers of the studs are 16 inches apart. What is the rut current in this state of affairs? Don't worry virtually one stud more or less.
For the human being body, what is the charge per unit of rut transfer by conduction through the body's tissue with the following conditions: the tissue thickness is 3.00 cm, the difference in temperature is ![]() , and the peel area is
, and the peel area is ![]() . How does this compare with the average heat transfer rate to the torso resulting from an energy intake of about 2400 kcal per 24-hour interval? (No exercise is included.)
. How does this compare with the average heat transfer rate to the torso resulting from an energy intake of about 2400 kcal per 24-hour interval? (No exercise is included.)
The rate of rut transfer by conduction is 20.0 Westward. On a daily basis, this is ane,728 kJ/mean solar day. Daily food intake is ![]() . And then only 17.2% of free energy intake goes as rut transfer past conduction to the environs at this
. And then only 17.2% of free energy intake goes as rut transfer past conduction to the environs at this ![]() .
.
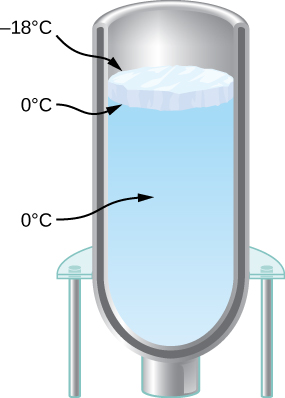
You have a Dewar flask (a laboratory vacuum flask) that has an open meridian and directly sides, as shown below. Yous fill it with water and put it into the freezer. It is finer a perfect insulator, blocking all oestrus transfer, except on the top. After a time, water ice forms on the surface of the water. The liquid water and the lesser surface of the ice, in contact with the liquid water, are at ![]() . The top surface of the water ice is at the same temperature as the air in the freezer,
. The top surface of the water ice is at the same temperature as the air in the freezer, ![]() Set the rate of estrus period through the water ice equal to the charge per unit of loss of heat of fusion every bit the water freezes. When the ice layer is 0.700 cm thick, discover the rate in m/south at which the ice is thickening.
Set the rate of estrus period through the water ice equal to the charge per unit of loss of heat of fusion every bit the water freezes. When the ice layer is 0.700 cm thick, discover the rate in m/south at which the ice is thickening.
An infrared heater for a sauna has a surface area of ![]() and an emissivity of 0.84. What temperature must it run at if the required power is 360 W? Fail the temperature of the environment.
and an emissivity of 0.84. What temperature must it run at if the required power is 360 W? Fail the temperature of the environment.
620 K
(a) Decide the power of radiation from the Sun by noting that the intensity of the radiation at the distance of Earth is ![]() . Hint: That intensity will be found everywhere on a spherical surface with radius equal to that of World's orbit. (b) Assuming that the Sun'due south temperature is 5780 Thousand and that its emissivity is 1, find its radius.
. Hint: That intensity will be found everywhere on a spherical surface with radius equal to that of World's orbit. (b) Assuming that the Sun'due south temperature is 5780 Thousand and that its emissivity is 1, find its radius.
Challenge Problems
Detect the growth of an ice layer equally a function of time in a Dewar flask as seen in (Figure). Call the thickness of the ice layer L. (a) Derive an equation for dL/dt in terms of L , the temperature T above the ice, and the properties of ice (which yous tin go out in symbolic form instead of substituting the numbers). (b) Solve this differential equation assuming that at ![]() , you have
, you have ![]() If you lot take studied differential equations, you lot will know a technique for solving equations of this type: manipulate the equation to become dL/dt multiplied past a (very simple) function of 50 on one side, and integrate both sides with respect to fourth dimension. Alternatively, you may be able to use your cognition of the derivatives of diverse functions to guess the solution, which has a elementary dependence on t. (c) Will the water eventually freeze to the bottom of the flask?
If you lot take studied differential equations, you lot will know a technique for solving equations of this type: manipulate the equation to become dL/dt multiplied past a (very simple) function of 50 on one side, and integrate both sides with respect to fourth dimension. Alternatively, you may be able to use your cognition of the derivatives of diverse functions to guess the solution, which has a elementary dependence on t. (c) Will the water eventually freeze to the bottom of the flask?
a. ![]() ; b.
; b. ![]() ; c. yes
; c. yes
Equally the very first rudiment of climatology, estimate the temperature of World. Assume it is a perfect sphere and its temperature is uniform. Ignore the greenhouse effect. Thermal radiations from the Sun has an intensity (the "solar abiding" Southward) of nigh ![]() at the radius of Earth'due south orbit. (a) Assuming the Sun'southward rays are parallel, what area must South be multiplied past to get the total radiation intercepted by Earth? Information technology volition exist easiest to respond in terms of Earth'due south radius, R. (b) Presume that Earth reflects about 30% of the solar energy it intercepts. In other words, World has an albedo with a value of
at the radius of Earth'due south orbit. (a) Assuming the Sun'southward rays are parallel, what area must South be multiplied past to get the total radiation intercepted by Earth? Information technology volition exist easiest to respond in terms of Earth'due south radius, R. (b) Presume that Earth reflects about 30% of the solar energy it intercepts. In other words, World has an albedo with a value of ![]() . In terms of S, A, and R, what is the rate at which Earth absorbs energy from the Sun? (c) Detect the temperature at which Earth radiates energy at the same rate. Assume that at the infrared wavelengths where it radiates, the emissivity e is 1. Does your upshot show that the greenhouse effect is important? (d) How does your answer depend on the the area of Earth?
. In terms of S, A, and R, what is the rate at which Earth absorbs energy from the Sun? (c) Detect the temperature at which Earth radiates energy at the same rate. Assume that at the infrared wavelengths where it radiates, the emissivity e is 1. Does your upshot show that the greenhouse effect is important? (d) How does your answer depend on the the area of Earth?
Let'due south stop ignoring the greenhouse issue and incorporate it into the previous problem in a very crude manner. Assume the atmosphere is a single layer, a spherical trounce around Earth, with an emissivity ![]() (called simply to give the right answer) at infrared wavelengths emitted by Globe and past the atmosphere. All the same, the atmosphere is transparent to the Dominicus'south radiations (that is, assume the radiation is at visible wavelengths with no infrared), so the Sun'south radiation reaches the surface. The greenhouse consequence comes from the departure between the temper's transmission of visible light and its rather strong assimilation of infrared. Note that the atmosphere's radius is not significantly different from Earth's, merely since the atmosphere is a layer in a higher place World, it emits radiation both up and downward, so it has twice World'due south area. In that location are three radiative energy transfers in this problem: solar radiation absorbed by Earth'south surface; infrared radiations from the surface, which is absorbed by the temper according to its emissivity; and infrared radiations from the atmosphere, half of which is absorbed past Earth and half of which goes out into infinite. Utilise the method of the previous problem to get an equation for Globe's surface and ane for the atmosphere, and solve them for the two unknown temperatures, surface and atmosphere.
(called simply to give the right answer) at infrared wavelengths emitted by Globe and past the atmosphere. All the same, the atmosphere is transparent to the Dominicus'south radiations (that is, assume the radiation is at visible wavelengths with no infrared), so the Sun'south radiation reaches the surface. The greenhouse consequence comes from the departure between the temper's transmission of visible light and its rather strong assimilation of infrared. Note that the atmosphere's radius is not significantly different from Earth's, merely since the atmosphere is a layer in a higher place World, it emits radiation both up and downward, so it has twice World'due south area. In that location are three radiative energy transfers in this problem: solar radiation absorbed by Earth'south surface; infrared radiations from the surface, which is absorbed by the temper according to its emissivity; and infrared radiations from the atmosphere, half of which is absorbed past Earth and half of which goes out into infinite. Utilise the method of the previous problem to get an equation for Globe's surface and ane for the atmosphere, and solve them for the two unknown temperatures, surface and atmosphere.
- In terms of Earth'southward radius, the constant
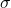 , and the unknown temperature
, and the unknown temperature 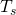 of the surface, what is the ability of the infrared radiation from the surface?
of the surface, what is the ability of the infrared radiation from the surface? - What is the power of Globe's radiations absorbed by the temper?
- In terms of the unknown temperature
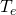 of the atmosphere, what is the ability radiated from the atmosphere?
of the atmosphere, what is the ability radiated from the atmosphere? - Write an equation that says the power of the radiation the atmosphere absorbs from Earth equals the power of the radiation it emits.
- Half of the ability radiated by the temper hits Earth. Write an equation that says that the ability World absorbs from the atmosphere and the Dominicus equals the power that it emits.
- Solve your 2 equations for the unknown temperature of Earth.
For steps that make this model less rough, come across for example the lectures by Paul O'Gorman.
a. ![]() ; b.
; b. ![]() ; c.
; c. ![]() ; d.
; d. ![]() ; eastward.
; eastward. ![]() ; f.
; f. ![]()
A Person Begins To Feel Cooler When A Breeze Moves Over The Skin. Which Mechanism Is Involved?,
Source: https://opentextbc.ca/universityphysicsv2openstax/chapter/mechanisms-of-heat-transfer/
Posted by: wiggspitions.blogspot.com


0 Response to "A Person Begins To Feel Cooler When A Breeze Moves Over The Skin. Which Mechanism Is Involved?"
Post a Comment